This is an INDEPENDENT website developed by long-time Emmy Award-winning Journalist Kym McNicholas.
This site is NOT in any way associated or affiliated with Ra Medical Systems or its DABRA Laser System
It depicts my journey following what I believe is a breakthrough medical device from FDA Clearance to commercialization to IPO...and beyond.
This is an INDEPENDENT website developed by long-time Emmy Award-winning Journalist Kym McNicholas.
This site is NOT in any way associated or affiliated with Ra Medical Systems or its DABRA Laser System
It depicts my journey following what I believe is a breakthrough medical device from FDA Clearance to commercialization to IPO...and beyond.
- INNOVATION:
Executing new ideas to create value
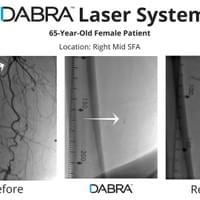
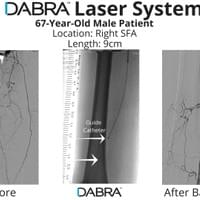
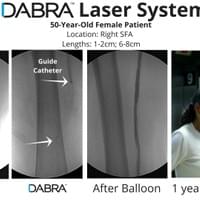
When it comes true innovation and you look at all of the greatest innovators of all time, they always picked up where others left off. The ones that have created the greatest value figured out where other devices were failing and they created the solution. In this case, all of the devices tackling artery blockages required a guidewire to aid them in navigating the vessel in order to clear a blockage. If the guidewire couldn't penetrate the lesion, they couldn't cross. They would have to figure out ways around it if it was even possible, -- even if it caused trauma to the vessel leading to bailout stenting. The next best option was invasive, costly bypass surgery and ultimately amputation.
More than 200,000 people have their legs amputated every year because of this problem.
That's where the market begins. That's why Ra Medical's entire DABRA FDA study focused on those patients where other devices failed.
Why create something and prove it can just do what every other device does?
True innovation goes above and beyond that.
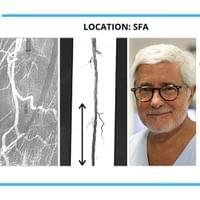
That's the benefit of this device. It's a device designed to cross Chronic Total Occlusions (CTOs) without the aid of a guidewire. The challenge was that doctors aren't used to it. In fact doctors are trained in school and during the residencies that the guidewire is their go-to device to navigate the vessels. Funny thing, is that not only do guidewires get them into trouble more often than not by entering into subintimal territory and guiding atherectomy devices with them, but also doctors for many years were able to actually navigate the human vasculature without the aid of the guidewire. The guidewire was developed mainly to overcome the tortuosity of the coronary region.
Even still, I've heard many naysayers over and over again that say it can't be done, or that it needed to be changed to be like the other devices.
It doesn't make sense to me that a doctor wouldn't at least try a device that could navigate the vessel using basic wire technique they already know and that is safer for patients.
Most will try it. And I've learned is that if they're properly trained and they see proof of smooth, non-turbalent flow in the final run-off with their own eyes, they quickly become believers.
Doctors I spoke with candidly expressed their skepticism they had prior to use and how they now view what seemed to be a disadvantage, or impossible, is actually to DABRA's advantage as well as to patients who couldn't be treated otherwise by minimally invasive means. The key is - seeing is believing....
Those are just a sampling of doctor interviews where their tone quickly changed following a procedure where DABRA cleared a blockage in which other devices failed and the next step would be invasive bypass or amputation.
I'm not kidding when I say that EVERY doctor I have encountered in this three year journey has told me,
"It can't work without a guidewire."
All the inventor would have to do is pull out his tablet filled hundreds of case videos such as the ones below and say, "Doctor, don't just take my word for it. See for yourself."
And after watching just a few short videos, the doctor would be ready to try DABRA.
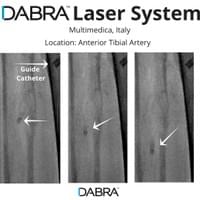
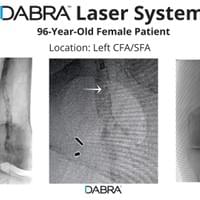
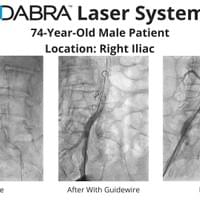
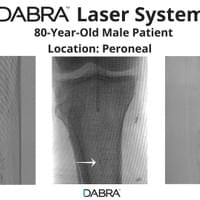
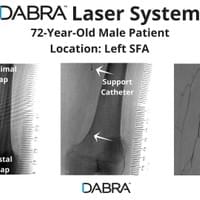
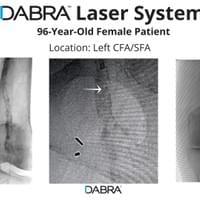
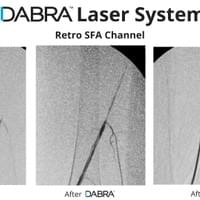
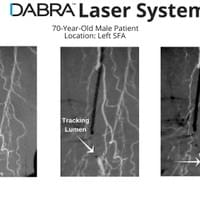
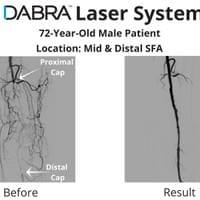
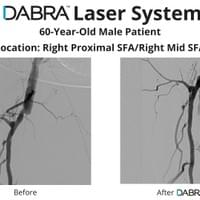
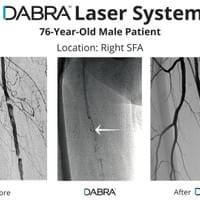
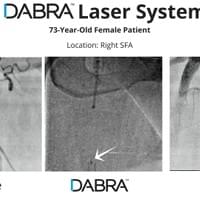
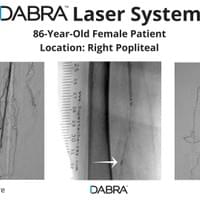
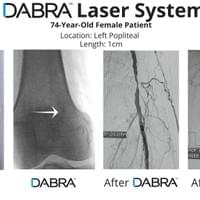
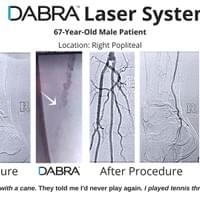
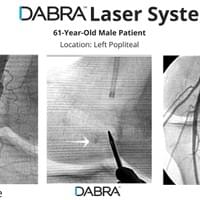
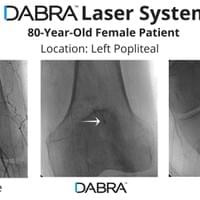
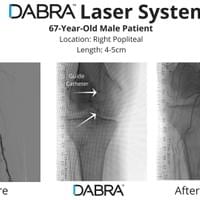
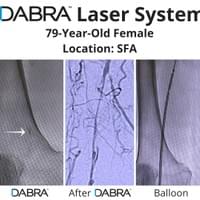
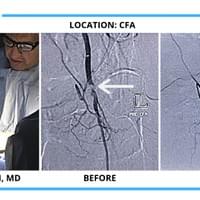
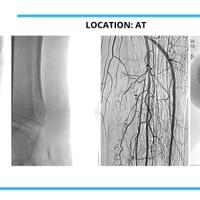
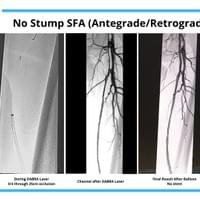
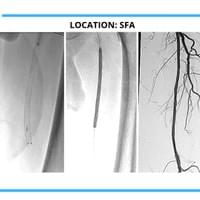
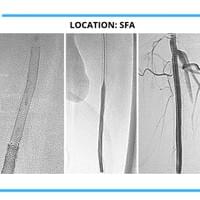
The cases below show the DABRA Catheter navigating the vessels without the aid of a guidewire.
"Are you still unsure doctor? Check out a few more with a guidewire."
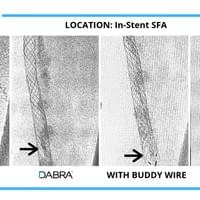
The cases below show the DABRA Catheter navigating the vessel alongside a guidewire without disrupting it. The guidewire is NOT aiding the DABRA Catheter in navigation, rather it's maintaining real estate already gained by DABRA in a previous pass through the vessel so the DABRA Catheter can increase lumenal gain.
The doctors would all say,
"Wow, if it can do what I see it does, then I'll try it. But there's no use in doing the cases I already can do with other devices."
Makes sense, right?
Well, it's definitely a tall order to expect that of any device.... to tackle the toughest patients.
Typically during a training you would think a doctor would want to start with the easier patients and ease up to the tougher cases.
But these doctors don't have the time or the money to waste.
Either the device works and is easy-to-learn/easy-to-use or they can't be bothered.
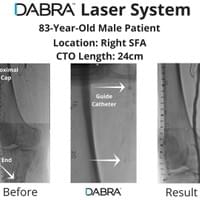
Obviously if the doctors are going straight for the impossible, there's an unmet need. Each one had cases in which they were at a loss at what other option they had other then to send the patient into invasive bypass or amputation.
Every doctor we visited from El Centro, California to Utrecht, Netherlands literally brought back patients in which other devices had failed and the DABRA would be their only hope to prevent bypass or amputation. Many of the patients were too sick for bypass. So, amputation would be the next step if DABRA didn't restore blood flow.
But DABRA was up for any challenge. In its FDA study, for DABRA to have successfully tackled only the toughest cases where other devices had failed with a 94% success rate with very little time for training, I had no doubt it would find the same success with doctors and their toughest cases beyond FDA clearance.
The inventor Dean Irwin could quickly get a doctor up-to-speed and and assist them in success after success in the toughest cases in which other devices continued to fail.
Doctors told me they prefer to clear blockages in the least invasive, least traumatic way possible. Not one doctor I spoke with ever wanted to sentence a person to amputation. Knowing DABRA would give them one last shot limb salvage, they were quick to want to try the device. Some barely even wanting to sit through training. They wanted to get right in the cath lab, scrub in, and put DABRA to use.
Why would they want to go through extensive training if the device didn't work?
With a 10 minute training overview such as this one, the doctors were off-and-running!
I captured this video in Hoxter, Germany. This was the doctor's second case of the day and he was already deploying, calibrating, and using the DABRA Catheter on his own without prompting.
You might wonder how it's possible for these doctors to use this device for the first time without much training. Engineers designed the DABRA Catheter to easily navigate through the twists and turns of the human vasculature. It was just a matter of the doctor getting used to a few nuances -- one being they don't have to work as hard. These physicians are accustomed to pushing and forcing vessels open with whatever was at their disposal. I saw them be very creative in trying to help their patients - even flipping guidewires around and using their sharp distal end to poke through tough, calcified lesions.
Their dedication was clear, but from my observation and discussions with them, obviously they needed a new tool, one that was less traumatic and more effective.
The trick to the success in using DABRA, was they needed to ease up, NOT push, and let the DABRA's photoablative properties do the work.
'
I've watched the inventor train doctors and offer step-by-step guidance and support through a procedure to clear blockages other devices couldn't and the proof is in the final run-off.
First time out of the gate...what I saw was remarkable - success after success!
The number of first-case success stories blew my mind.
FIRST TIME USER SUCCESS STORIES
LIVE CANDID REACTION DURING THE CASE FOLLOWING DABRA USE
"Beautiful case!"
"It's a smooth channel!"
"WooHoo, lookie lookie!"
"I think we can consider this a good success."
CANDID REACTION DIRECTLY FOLLOWING PROCEDURES USING DABRA
"I think we can consider this a good success."
"This was a good feeling for us!"
"Very nice. No dissection. Very nice result."
"With this instrument we can perform recanalization fast and easy."
- TRAINING
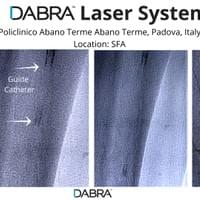
The list of first-time success stories goes on and on. I captured hundreds of cases around the world with sometimes a new doctor every day for 9 straight days driving across Italy introducing this technology to hospital after hospital, for example. The success stories abound.
Just because a doctor loved the device and saw it's benefit, it didn't always translate into a sale.
You would think that just the thought of being able to treat those 200,000 patients a year in which a guidewire may have been the culprit as to why they are now lined up for amputation, doctors would do everything in their power to learn the limb-saving technique. Even if there was just a slight chance this device would work, wouldn't you think that a doctor would want a device such as this even on-hand just in case other devices failed and prevent sentencing their patient to life in a wheelchair or even worse, death in the next two years as the mortality rate following major amputations is high for vascular disease patients?"
It seemed logical to me. But I discovered that overseas, there are a lot of administrative hurdles and therefore it takes a lot of time to bring in new technologies.
In the U.S., I discovered large companies with competing technologies who finance cath labs hindered adoption, some sales reps with strong personal relationships with doctors hindered adoption, competitors deliberately sabotaging the sale telling lies to the doctors saying it was not reimbursable, hindered adoption, and just simply unwilling to break current habits as they're comfortable with their current tools and techniques even if they don't have as high of a success rate and patients return regularly due to restenosis, hindered adoption.
The top hinderance to adoption, however, was training.
While it was easy to get a doctor started, and get their first success, it would take about half a dozen cases with a trainer and the successful training methods deployed before the doctor was off-and-running on his/her own. His methods worked with every single doctor I observed over the course of three years traveling around the world with thousands of hours captured on camera in the cath lab. The doctors were certainly not experts by any means at that point. But they were at least comfortable with the device and were excited to replicate the proven results -- smooth flow in the final run-off.
They were so comfortable that they started sending me and the inventor videos documenting their cases, some even narrated. This one was from Dr. Raghotham Patlola who participated in the company's FDA study. He was off-and-running after about half-a-dozen cases. He did this one on his own.
One of the challenges with this type of disruptive technology is being able to change the habits of the physicians. Once again, they're used to the aid of a guidewire to navigate the vessel and pushing really hard. I watched the inventor spend hours alongside the doctor working with physicians helping them to get the most out of the device and discover its potential.
What I saw was a strategy to achieve this by
* Gaining the trust of the physician by being technically and clinically competent
* Building confidence of the physician and the staff by demonstrating the device, and showing actual procedures
* Providing support by teaching the methods and techniques to break those old habits and become proficient
* Educate by teaching the science and the benefits of the device and the techniques.
These methods worked. Again, proof is a 94% success rate with NO adverse events in their FDA study. Throughout the study, the technology worked on 60 out of 64 patients! It recanalized 75 out of 83 lesions (100% blockages) that could not be crossed with a standard guidewire. Every vessel in the legs was represented in the study, both above- and below-the-knee. And multiple approaches were used from the most typical contralateral cross-over technique to tibial access or from the dorsalis pedis. Those results were not typical with comparative devices.
Beyond the study, I observed hundreds of other cases in the U.S. and around the world in which the inventor of this technology was able to successfully train doctors. See some of the successful methods he used below which led to success after success.
BUILD CONFIDENCE
PROVIDE SUPPORT
From Deployment...
...through results.
This training program, when implemented, has proven successful time and time again. Case in point is just in 2019 alone, I traveled to Germany, France, Italy, and the Netherlands observing dozens of cases --- even 8-10 in one day in Cailais, France. We then roadtripped through Southern and Central, California performing cases from Bakersfield to Fresno.
All were a success using the training methods above.
Educate
Example:
After a doctor has performed several successful cases, they want to learn more about the technology and how it's able to do what other devices can't do. I was able to capture this spontaneous conversation between Dean Irwin and Dr. Athar Ansari after a case in which doctors at a large tertiary center had failed several times. Even Dr. Ansari wasn't sure it could be possible. The DABRA Laser System was successful and that sparked his intense interest in getting to the bottom of exactly how and why it works.
LIVE Cases
Doctors are so confident inn the technology that they're willing to share their success using DABRA with their peers performing a LIVE case or multiple cases in front of hundreds of other doctors attending popular medical conferences. If they weren't confident that the device would calibrate and effectively treat their patients, they wouldn't agree to put their reputation on the line.
© 2018